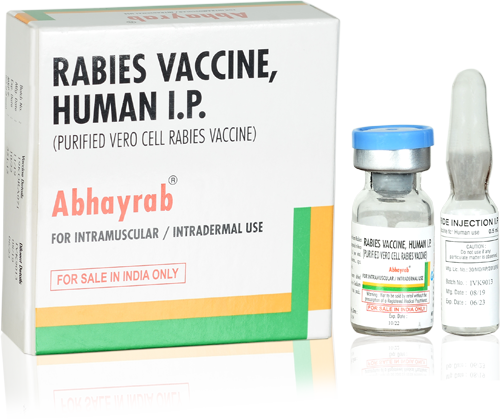
India's No. 1 Anti Rabies Vaccine
Saving Millions of lives every year
Vaxtar-5 Product Detail
- Dose administration
- Indications
- Adverse effects
- Warnings and Special precautions
- Interaction with other medications
- Contraindications
For Intramuscular Vaccination:
In infants and pre-school children Vaxtar-5 is administered intramuscularly at a dose of 0.5mL with an interval of four weeks between doses starting at six weeks of age. In some countries, where the risk of perinatal HBV infection is high, the first dose of hepatitis B vaccine is given immediately after birth. A booster dose of DTP and Hib is administered at the age of 15 to 18 months. A reinforcing booster dose of DTP can be administered at the age of five years.
Vaxtar-5 should be administered at other site if co-administered with other injections.
This vaccine is widely indicated against diphtheria, tetanus, whooping cough, hepatitis B, and Hib infections.
Administration of vaxtar-5 may cause certain common side effects, such as:
- Pain, swelling, warmth, and redness at the site of injection
- Fever
- Irritability
- Unusual crying
- Loss of appetite
- Vomiting
- Drowsiness
Rarely, vaxtar-5 may cause certain neurological complications like cochlear lesion, brachial plexus neuropathies, encephalopathy, and paralysis. Prolonged convulsions is the severe neurological side effect associated with vaxtar-5.
- Vaxtar-5 would not be effective if the child is already exposed to hepatitis B virus for long-term.
- Inform the doctor if the child has experienced fever (>40.0°C), shock, persistent crying, and convulsions with or without fever within 48 hours after the administration of vaxtar-5.
- Children with any coagulation disorders, such as thrombocytopenia are ineligible for vaxtar-5, unless the potential benefits outweighs the risks.
- Infants or children with a family history of convulsions are at higher risk of neurological events and permanent neurological damage than children without such history.
- Precautions to prevent the risk of adverse events associated with this vaccine must be taken before receiving this vaccine.
- The parents must inform about their family history and health status of the child to the doctor before going for vaccination.
Vaxtar-5 must be used with caution in patients who are taking anti-coagulants, immunosuppressants, corticosteroids, and anti-cancer drugs.
Vaxtar 5 is contraindicated if:
- Hypersensitive to any components present in the vaccine
- Anaphylactic reaction with a previous dose
- Encephalopathy
- Neurological condition
Vaxtar-5 should be avoided in infants and children with severe febrile illness until recovery. However, children with minor conditions, such as upper respiratory tract infections with or without low-grade fever can receive this vaccine.
Our Initiatives
Frequently Asked Questions (FAQS)
Rabies virions are bullet-shaped with 10-nm spike-like glycoprotein peplomers covering the surface. The ribonucleoprotein is composed of RNA encased in nucleoprotein
Rabies virions are bullet-shaped with 10-nm spike-like glycoprotein peplomers covering the surface. The ribonucleoprotein is composed of RNA encased in nucleoprotein
Rabies virions are bullet-shaped with 10-nm spike-like glycoprotein peplomers covering the surface. The ribonucleoprotein is composed of RNA encased in nucleoprotein
Rabies virions are bullet-shaped with 10-nm spike-like glycoprotein peplomers covering the surface. The ribonucleoprotein is composed of RNA encased in nucleoprotein
Contact Us
By clicking on the submit button above, I hereby consent to a Human Biologicals Institute contacting me for providing me further information on Abhayrab. I also consent to the Terms of Use and Privacy policy of Human Biologicals Institute and confirm that I am a registered medical practitioner residing in India and the information submitted with this form is true and correct. hbi@indimmune.com
Follow Us
Disclaimer: The data contained in this website is intended for general reference only and is meant for viewers in India only. It may refer to pharmaceutical products, diagnostic techniques, therapeutics or indications not yet registered or approved in a given country and it should be noted that, over time, currency and completeness of the data may change. For updated information, please contact the Company. This data should not be used to diagnose, treat, cure or prevent any disease without the professional advice of a Registered Medical Practitioner, and does not replace medical advice or a thorough medical examination. Registered Medical Practitioners should use their independent professional judgement in checking the symptoms, diagnosing & suggesting the appropriate line of treatment for patients. Human Biologicals Institute (HBI) is not in any way influencing, propagating or inducing anyone to buy or use HBI products. HBI accepts no liability for any loss, damage or compensation claims in connection with any act or omission by anyone based on information contained in or derived through use of this website. Duplication of or copying any data requires prior permission of the copyright holder. For adverse event reporting with respect to products of HBI, kindly contact our local Drug Safety Unit at hbi@indimmune.com.